
Progesterone receptor-negative tumors in the arms were 17.6%, 15.7%, and 16.3%, prospectively. Rate of cT1 tumors was 50.4%, 48.8%, and 46.5%, in arms A, B, and C, respectively cN0 tumor rates were 71.4%, 75.6%, and 70.5%, respectively. The median ages in the arms ranged from 50 to 51.5 years, and investigators said the characteristics were well balanced between the 3 groups.
#T dm1 breast cancer plus#
The intent-to-treat population included 119 patients randomly assigned to T-DM1 (3.6 mg/kg body weight every 3 weeks for 4 cycles Arm A), 127 randomly assigned to T-DM1 (same regimen) plus endocrine therapy (Arm B), and 129 randomly assigned to trastuzumab (loading dose of 8 mg/kg and then 6 mg/kg every 3 weeks for 3 more cycles) plus endocrine therapy (Arm C). Investigators enrolled 375 patients with early HER2-positive/HR-positive breast cancer at 48 centers from October 2012 to March 2015.

#T dm1 breast cancer trial#
“Our trial results add to the existing evidence that HER2-positive early breast cancer may require approaches that differ regarding the treatment of the luminal (HR-positive) and the nonluminal (HR-negative) subtypes, “added Harbeck et al. T-DM1 may be an efficient and safe alternative for patients who are not suited for systemic chemotherapy in this setting the addition of endocrine therapy does not seem to play a crucial role,” first author Nadia Harbeck, MD, University of Munich, and coinvestigators wrote. “Only 4 cycles of neoadjuvant T-DM1 in HER2-positive/HR-positive early breast cancer yield substantial pCR rates-quite comparable to standard chemotherapy plus trastuzumab (or even to dual HER2 blockade). Of those, 35.7% achieved a pCR compared with 19.8% in nonresponders (odds ratio, 2.2 95% CI, 1.24-4.19 P =.

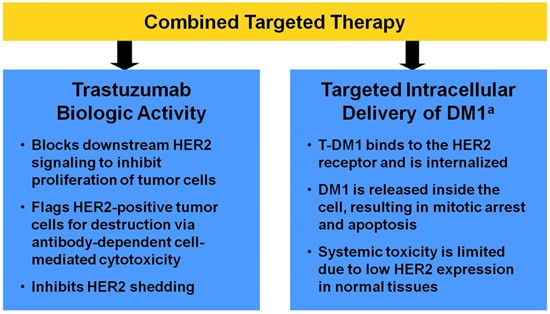
Two-thirds of patients who responded were early responders. In contrast, 15.1% of patients assigned to trastuzumab and endocrine therapy had a pCR ( P <.001).Įarly response was defined as a ≥30% decrease in the Ki-67 level after a single therapy cycle. In the prospective, neoadjuvant phase II ADAPT trial conducted by the West German Study Group, pCR was 41.0% for patients assigned to T-DM1 alone and 41.5% for those who received T-DM1 and endocrine therapy. Twelve weeks of neoadjuvant T-DM1 (ado-trastuzumab emtansine Kadcyla) with or without endocrine therapy induced superior pathologic complete response (pCR) compared with trastuzumab (Herceptin) plus endocrine therapy in patients with HER2-positive/HR-positive early breast cancer, according to findings recently published online in the Journal of Clinical Oncology.


 0 kommentar(er)
0 kommentar(er)
